QUESTION:
Most of the zirconia crowns and fixed prostheses coming from my labs lately are too light in color, despite my providing preoperative photos and descriptions to the technicians. I have tried several different laboratories, and I am still frustrated with the mismatched colors. Although zirconia crowns are strong, I believe their color-matching is severely lacking. My colleagues have expressed similar color mismatch as well. What can be done to improve this obvious problem?
ANSWER:
I agree completely with your criticism of the current generation of zirconia crowns. The Technologies in Restoratives and Caries Research (TRAC) Division of the Clinical Research Foundation has accomplished long-term in vivo research on zirconia crowns and found their strength to be fantastic and their service over many years to be outstanding. However, only recently has the color of zirconia crowns been improved so they are more acceptable.
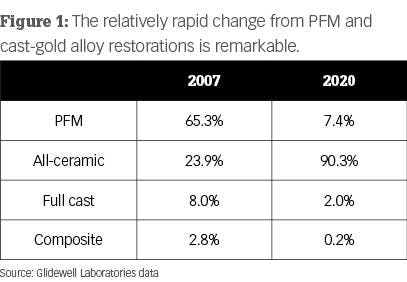
The changes to improve color and translucence of the crowns has been successful, but it also has had some negative effects. The information in Figure 1 is from Glidewell Laboratories and shows remarkable changes over the last several years as ceramic restorations have become mainstream. What are manufacturers doing to improve the esthetics of zirconia restorations? To answer that question, let’s discuss the various types of zirconia.
Zirconia and its various forms
When observing the 2020 data from Glidewell Laboratories in Figure 1, it is obvious that zirconia wins the popularity contest. As you probably know, Glidewell started this clinical revolution with BruxZir, which soon dominated the market. Zirconia-based crowns have been available for more than 21 years, and full-zirconia crowns have been available for about 10 years.
In the first few production years, the original zirconia-based crowns had challenges, but since then those problems have largely been overcome. Despite that, the use of zirconia-based restorations has not grown markedly.
Full-zirconia restorations of various formulations are extremely popular and state-of-the-art. But zirconia is not zirconia. You are undoubtedly familiar with the ADA/ISO classification of ceramic crowns, which we previously published in the July 2019 issue of Clinicians Report. (Visit cliniciansreport.orgto read the article in detail.)
The initial BruxZir formulation was classified as class 5 tetragonal zirconia. This is probably what you will receive if you order class 5 or 3Y (3 molar percent yttrium oxide) tetragonal zirconia. This formulation has been used in nondental applications in various industries for more than 30 years. It is the strongest form of zirconia and has optimal transformation toughening (lack of crack propagation) during service. Unfortunately, this is the iteration of dental zirconia most difficult to make esthetically acceptable.
If you are planning a long-span fixed partial denture (FPD) or treating a patient with bruxism, this is an excellent choice shown to be comparable in strength to porcelain-fused-to-metal (PFM) in our long-term TRAC studies. It can be made esthetically acceptable for some situations by presinter staining or optimum use of external stains.
Manufacturers have been experimenting with making 3Y zirconia more esthetic. The most successful and easiest method to date is to increase the translucence by adding more translucent material to the zirconia. ADA/ISO classifies this type of zirconia as class 4 cubic-containing zirconia. It is also identified as 4Y or 5Y or combinations of those percentages. Although additional long-term clinical research is still needed for the so-called esthetic zirconia, class 4 zirconia currently appears to be adequate for singles, short-span FPDs, and other situations not requiring the strength of 3Y zirconia.
Why do you need to know the zirconia classifications?
Your knowledge of these classifications allows you to communicate with salespeople and laboratory technicians, as well as understand what materials you are putting into your patients’ mouths. Knowing this, you’ll better understand the changes that are being made in the original dental zirconia restorations.
Adding more translucent materials to the zirconia. Additional oxides are now being added to the original Glidewell formulation of zirconia (3Y, class 5 zirconia versions) by many manufacturers. This formulation change improves translucency and esthetics. However, the change to 4Y or 5Y or combinations of those reduces the overall strength of zirconia and its ability to “heal” microcracks. (Higher transformation toughening increases the lack of subsequent crack propagation.) Although this procedure reduces strength, clinical success is currently being proven and reported in the field by practicing dentists. The esthetic characteristics of some of these class 4 restorations can rival IPS e.max (Ivoclar Vivadent).
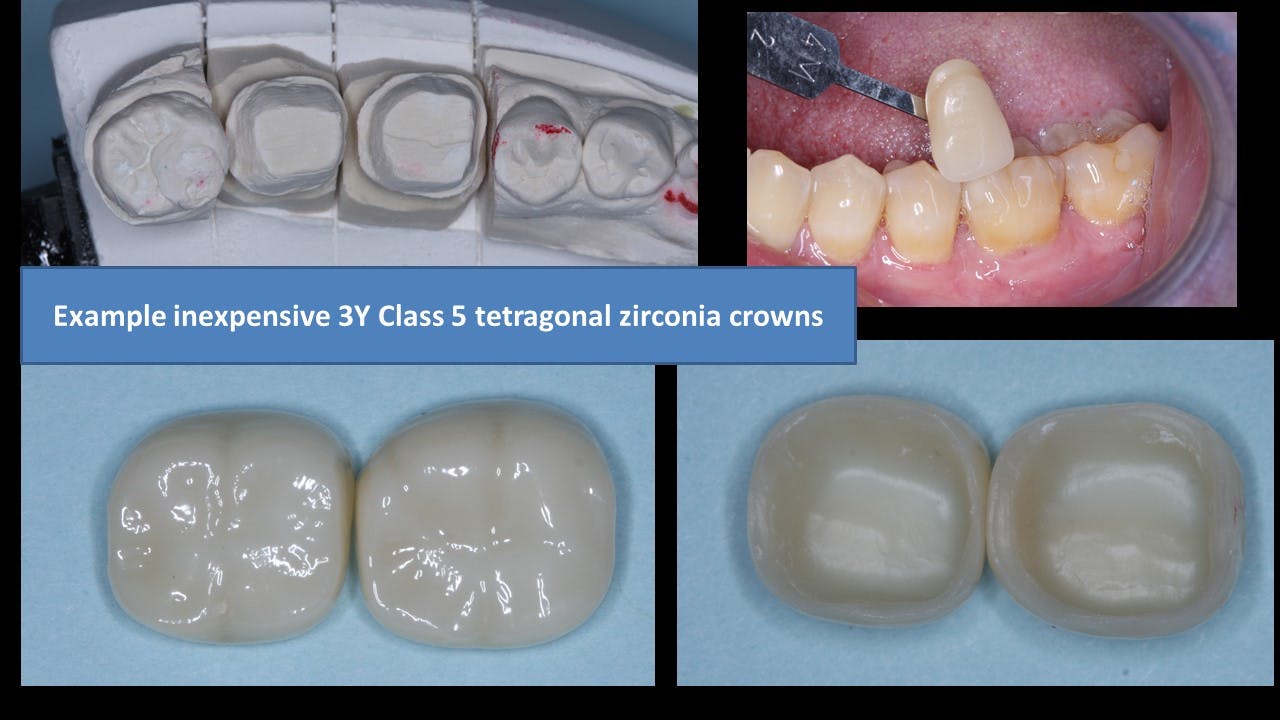
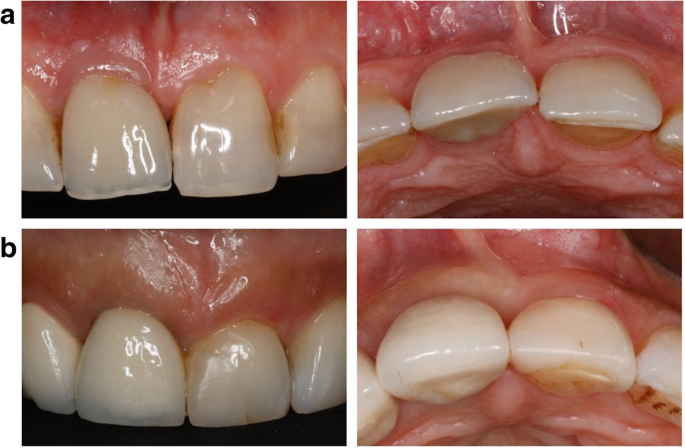
Placing relatively thick layers of stain and glaze on the external of the restorations. Many labs are firing ceramic on the outside of the mismatched zirconia to improve the zirconia color (figure 2). In our research, these additions are usually feldspathic ceramic and are showing wear on the opposing teeth. There is also a slow but continuing loss of the added superficial layers. If the external layers are thin, the result is a slow change back to the original zirconia colors.
Internal staining of zirconia. Some labs are staining the 3Y, class 5 zirconia in the presintered stage, which improves the color significantly. However, this requires artistic technicians, more time, and a greater cost to the dentist (figure 3).
Currently, most indirect restorations are ceramic, and that trend will undoubtedly continue to grow. Metal and porcelain-to-metal restorations are showing a relatively rapid decrease in use in dentistry.
Is there a dental laboratory problem?
Yes! The laboratory industry has changed rapidly and significantly. Until recently, porcelain-fused-to-metal restorations were the norm. That situation then changed to nearly all ceramic restorations, with zirconia restorations being rapidly produced by milling machines that use computer-driven software and by lithium disilicate being pressed or milled.
Many artistic laboratory technicians are being replaced with highly competent, computer-savvy technicians who produce restorations at record speeds considered to be impossible by older technicians. As a result, restorations are less expensive but often less esthetically acceptable.
If needed, clinicians must seek artistically oriented laboratory technicians to accomplish the complex esthetic results desired for some patients, and consequently, we must expect to pay higher fees for these restorations. Esthetic restorations are available—certainly with lithium disilicate (IPS e.max) and, with effort, even zirconia.
Which indirect restorations are desirable and for what situations?
I have heard some dentists comment with mixed opinions on the esthetic dilemma about which you asked. Most dentists admit, to an embarrassing degree, that many zirconia restorations do not adequately match adjacent teeth, but they further comment that zirconia restorations in the posterior nonesthetic regions are far more esthetically acceptable than gold alloy or porcelain-fused-to-metal when the glaze and staining have worn off.
Porcelain-fused-to-metal
Don’t forget the 70-year success of these restorations, especially when patients have had proven success with previous PFM restorations in their mouths. The advantages and disadvantages of these restorations are well known. Many labs can make highly esthetic PFM restorations. They are especially useful in clinical situations requiring precision or semiprecision attachments, which are not currently available for zirconia or lithium disilicate restorations. An additional continuing use for PFM is long-span fixed prostheses. Although not as commonly used as in the past because of the availability of implants, PFM restorations are still occasionally needed.
Lithium disilicate (most common brand name IPS e.max)
Can you name any other proven successful type of indirect restoration that equals the superior esthetic result of e.max for single-tooth restorations? That question is easy to answer. Many crown types have been tried and were initially successful, but they failed the need for longevity. The success of e.max in selected three-unit anterior FPDs has been shown, but I suggest that the newer generations of zirconia (class 4 zirconia) will probably prove to be more successful for long-term clinical success in these anterior situations.
Summary
The crown revolution has changed almost every treatment plan that requires crowns or fixed prostheses. This article describes the state-of-the-art and makes suggestions about which materials to use, their advantages and limitations, as well as how to produce the esthetic result necessary for specific clinical situations.
Author’s note: The following educational materials from Practical Clinical Courses offer further resources on this topic for you and your staff.

Recent Comments